
VIASURE Real Time PCR Detection Kits
Respiratory Panel 06
Description
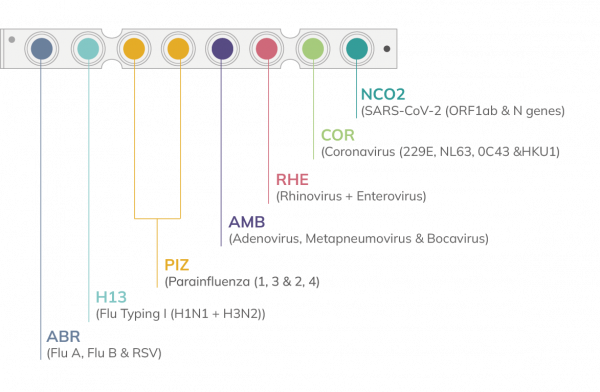
VIASURE Respiratory Panel VI Real Time PCR Detection Kit is designed for the specific identification and differentiation of Influenza A (Flu A), Influenza B (Flu B) and/or Human Respiratory Syncytial Virus (RSV); Influenza A (H1N1)pdm09 and H3N2 subtypes; Parainfluenza 1 (PIV-1), Parainfluenza 2 (PIV-2), Parainfluenza 3 (PIV-3) and/or Parainfluenza 4 (PIV-4); human Adenovirus (AdV), Metapneumovirus (MPV) and/or Bocavirus (BoV); Human rhinovirus (HRV) and/or human enterovirus (HEV); and/or ); Coronavirus (CoV) 229E, NL63, OC43 and/or HKU1 strains; and/or SARS-CoV-2 in respiratory samples from patients with signs and symptoms of respiratory infection.
This test is intended for use as an aid in the diagnosis of Flu A, Flu B and/or RSV; Influenza A (H1N1)pdm09 and/or H3N2 subtypes; Parainfluenza 1, Parainfluenza 2, Parainfluenza 3 and/or Parainfluenza 4; AdV, MPV and/or BoV, HRV and/or HEV; CoV 229E, NL63, OC43 and/or HKU1 strains; and/or SARS-CoV-2 in combination with clinical and epidemiological risk factors.
RNA/DNA are extracted from clinical specimens, multiplied using Real Time amplification and detected using specific primers and a fluorescent reporter dye probe for Flu A, Flu B and RSV; Influenza A (H1N1) pdm09 and H3N2 subtypes; Parainfluenza 1, Parainfluenza 2, Parainfluenza 3 and Parainfluenza 4; AdV, MPV and BoV, HRV and HEV; CoV 229E, NL63, OC43 and HKU1 strains; and SARS-CoV-2.
Specifications
Information
Respiratory infections are a major global health problem, mainly affecting young children and the elderly in low- and middle-income countries. The causative agents of this type of infections are viral or bacterial, being viruses more frequently involved. The management of the infections is crucial to prevent epidemics or pandemics, so accurate and specific diagnosis tools are required.
