Certest Rapid Test
Frequently asked questions
Do you need help with any of our products or have an urgent question? Click on one of the following sections to find the answer.
Our tests are generally designed for professional use.
Only tests identified as self-testing can be used by non-professionals
A rapid test is a diagnosis technique that detects the presence or absence of a specific antigen in a few minutes. These antigens are unique for each pathogenic microorganism, so rapid tests detect the presence or absence of different microorganisms. There are also rapid tests that detect biomarkers. These are proteins whose presence or absence are related to the development of different diseases. Consequently, rapid tests allow a rapid screening of a wide range of diseases, both infectious and non-infectious.
Rapid tests are based on an antigen-antibody reaction. These reactions occur naturally in our bodies, and we can replicate them in our devices thanks to biotechnology. In this reaction, an antibody recognizes specifically the antigen we want to detect. After placing the sample in the test, if the sample contains the antigen we want to detect, the antigen-antibody reaction will occur, and a colored line will appear in the “T” area of the test. That indicates that the sample is positive.
In diagnosis, the accuracy of a test is defined as the harmony between the result of the test and the result offered by a model test. It is closely related to reproducibility, that is the ability of the test to offer similar results in similar conditions repeatedly.
Accuracy is determined by sensibility and specificity. Sensibility is the probability of an ill person to be diagnosed as positive in a rapid test whereas specificity is the probability of a wealthy person to be diagnosed as negative in a rapid test. Values of sensibility and specificity can vary depending on the analyte, the antibody, the brand… You can find the values of sensibility and specificity of all our products in the instructions that are provided with the tests.
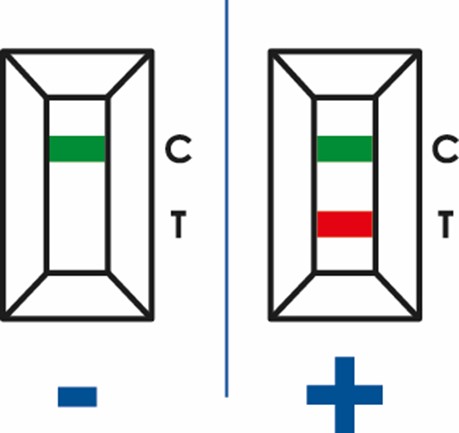
Results of rapid tests are determined by the appearance of different colored lines in the test membrane. Usually, tests have two different lines. The control line, that appears next to letter “C” of the device. This control line must always appear in the test, independent of whether the result is positive or negative, as it indicates that the test is working properly. The second line appears next to the “T” site, and it will only appear if the result is positive, that means that the analyzed sample contains the antigen we want to detect.
There are some tests that can detect the presence of multiple antigens at the same time. In these tests, there are several lines, identified as T1, T2, T3 etc. Each line is independent and detects the presence of a single antigen.
Interpretation of the results must be carried out within the test time, indicated in the instructions provided with the kit (usually 10’-15’). Once this time has passed, results are no longer valid.
If the test was carried out at hospital or at your medical centre, your doctor will indicate the corresponding protocol. If the test was carried out at home, take measures to prevent contagion if the illness is infectious and arrange a date with your doctor as soon as possible.
You must check that the test has not exceeded its expiration date and that it has been in proper humidity and temperature conditions (2-30ºC). Tests must remain in the sealed pouch until use. To carry out the test, you must follow the instruction provided with the kit.
They are different diagnostic techniques. Rapid tests are based on an antigen-antibody reaction, whereas PCR is based on DNA amplification by polymerases. Each technique offers different benefits and depending on the goal, you must choose one or another.
Benefits of rapid tests include: a fast and reliable result in a few minutes, the absence of specific and expensive material and they are easy to carry out.
Rapid tests are designed to be used with the general population without risks. In addition, you can consult the instructions for use to check possible interferences with other diseases or pharmacological treatments.
CERTEST has a wide range of distributors worldwide. As a final user, if you want to purchase our products, you can contact our local distributors or you can contact us for information about our distributor in your country.
As a company, if you want to become a CERTEST distributor, you can contact us via the information provided on our web page.
Our tests are designed to offer a reliable result in the test time indicated on the instructions (usually 10’-15’). Once this time has passed, results are not valid anymore.
CERTEST offers rapid test products for a wide range of diseases. Our tests detect pathogenic microorganisms (both virus and bacteria) that cause gastrointestinal and respiratory diseases, by using samples from different origins. We also have rapid tests for an early screening of some non-infectious diseases, like colorectal cancer (with tests like FOB and transferrin) and inflammatory bowel disease (with our calprotectin test).
For further information, you can consult our web page section about our rapid test products.
Our tests are designed to be carried out in laboratories, hospitals and medical centres by professionals, to assure a correct realization of the test and to obtain a reliable result.
Tests must be stored in places with low humidity and a temperature between 2-30ºC. In addition, tests must remain in the sealed pouch until use. Tests must not be frozen under any circumstance.
No, tests must not be used beyond their expiring date, because a reliable result cannot be guaranteed. The expiring date must always be checked before using the test.
CERTEST has a wide range of distributors worldwide. As a final user, if you want to purchase our products, you can contact our local distributors or you can contact us for information about our distributor in your country.
As a company, if you want to become a CERTEST distributor, you can contact us via the information provided on our web page.
CERTEST quality system is certified by ISO 13485 and MDSAP (ISO 13485:2016) and all our products have CE quality certification. In addition, considering the necessities of the markets of our distribution network, our products are locally registered.
What is more, we are in the process of updating our products accordingly to the new IVDR requirements.
You can contact us by email; by clicking on our web page section “contact” or you can use the following phone number (+34) 976 520 354. You will be assisted by our workers as soon as possible.
