
VIASURE Viral Particles
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit
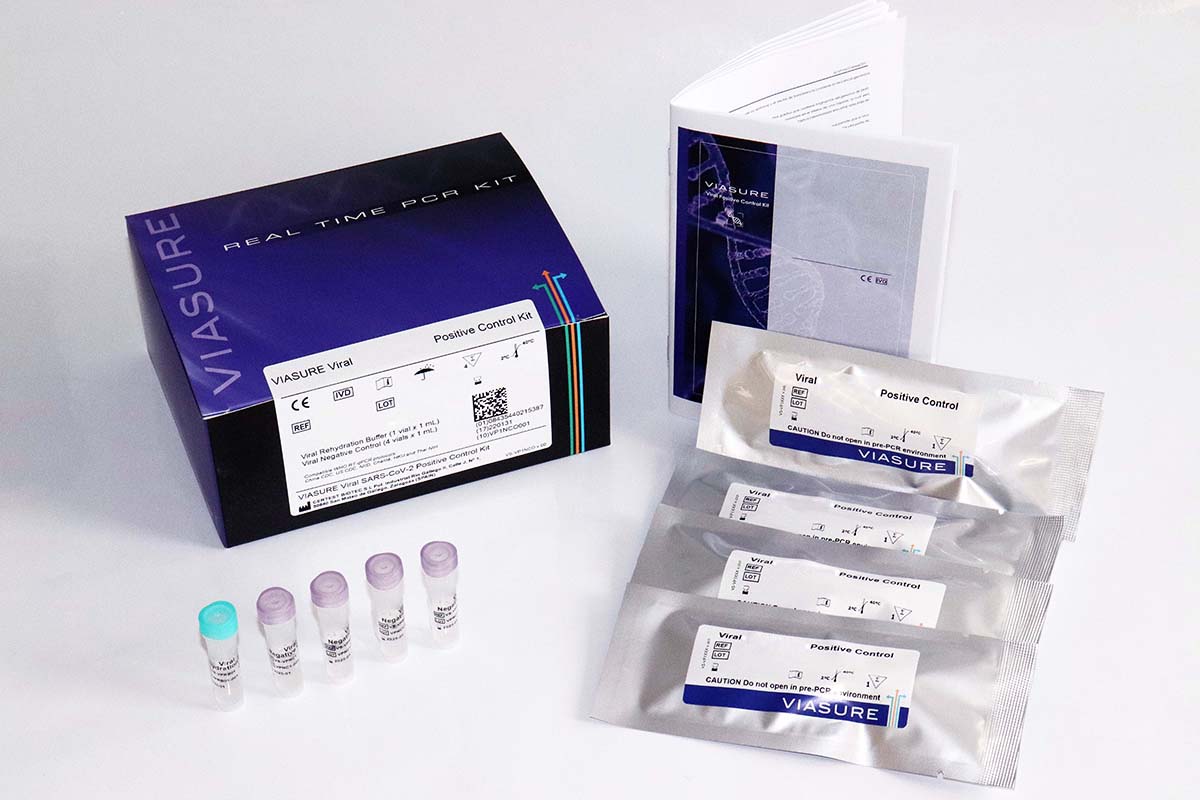
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit is intended for monitoring the entire Real Time PCR process, from nucleic acid extraction to amplification, suitable for any RT-qPCR protocol designed for the detection of influenza A and influenza B viruses and those RT-qPCR protocols designed for the detection of the M, N, F and L genes of respiratory syncytial virus (RSV).
Description
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit is intended for monitoring the whole reverse transcription quantitative real-time PCR (RT-qPCR) process, from nucleic acid extraction to reverse transcription and amplification steps.
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit contains non-replicative and non-infectious Influenza A, Influenza B and Respiratory Syncytial Virus (RSV subtype A) viral particles that are expected to be processed along with respiratory samples from individuals with clinical suspicion of Influenza A, Influenza B and RSV infection.
This Kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in molecular techniques and in vitro diagnostic procedures.
